Design and synthesis of N1,N8-diacetylspermidine analogues having a linker with desired functional groups†
Abstract
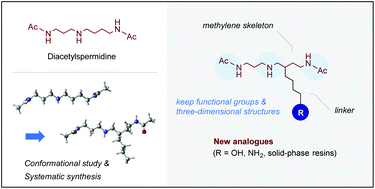
The synthesis of new N1,N8-diacetylspermidine (DiAcSpd) analogues having a linker with desired functional groups in the methylene skeleton, which have been designed by theoretical calculations, is described. We have also achieved the preparation of DiAcSpd supported on solid-phase resins, which have the potential to be used for the evolution of ligands by exponential enrichment (SELEX).


 Please wait while we load your content...
Please wait while we load your content...