Tetrafluoropyridyl (TFP): a general phenol protecting group readily cleaved under mild conditions†
Abstract
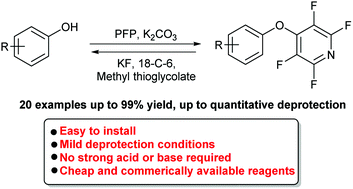
Phenols are extremely valuable building blocks in the areas of pharmaceuticals, natural products, materials and catalysts. In order to carry out modifications on phenols, the phenolic oxygen is routinely protected to prevent unwanted side reactions. Presently many of the protecting groups available can require harsh conditions, specialist equipment, expensive or air/moisture-sensitive reagents to install and remove. Here we introduce the use of the tetrafluoropyridyl (TFP) group as a general protecting group for phenols. TFP can be installed in one step with no sensitivity to water or air, and it is stable under a range of commonly employed reaction conditions including acid and base. The TFP protecting group is readily cleaved under mild conditions with quantitative conversion to the parent phenol, observed in many cases in less than 1 hour.
- This article is part of the themed collections: Synthetic methodology in OBC and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...