HUSY zeolite-promoted reactions of trifluoromethylated propargyl alcohols with arenes: synthesis of CF3-indenes and DFT study of intermediate carbocations†
Abstract
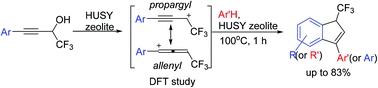
The reaction of CF3-propargyl alcohols [ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OH)CF3] with arenes under the action of acidic HUSY zeolite CBV-720 was investigated. It was found that the reaction at 100 °C for 1 h gave rise to 3-aryl-1-CF3-indenes in up to 83% yield. Electronic characteristics of the reaction key intermediates, mesomeric CF3-propargyl-allenyl cations [ArC
CCH(OH)CF3] with arenes under the action of acidic HUSY zeolite CBV-720 was investigated. It was found that the reaction at 100 °C for 1 h gave rise to 3-aryl-1-CF3-indenes in up to 83% yield. Electronic characteristics of the reaction key intermediates, mesomeric CF3-propargyl-allenyl cations [ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−HC+(CF3) ↔ ArC+
C−HC+(CF3) ↔ ArC+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CF3)], were studied by DFT calculations. A plausible cationic reaction mechanism is discussed.
CH(CF3)], were studied by DFT calculations. A plausible cationic reaction mechanism is discussed.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...