Nickel-catalyzed syn-stereocontrolled ring-opening of oxa- and azabicyclic alkenes with dialkylzinc reagents†
Abstract
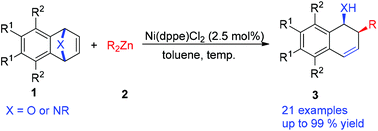
A new nickel-catalyzed syn-stereocontrolled ring-opening of oxa- and azabicyclic alkenes with dialkylzinc reagents was developed, which afforded the corresponding cis-2-alkyl-1,2-dihydronaphthalen-1-ols and 1,2-alkyl amide derivatives in moderate to excellent yields (up to 99% yield) under mild conditions. In this work, we successfully avoided obtaining hydride addition byproducts arising from β-hydride elimination on an ethyl nickel species. Furthermore, a plausible mechanism for the ring-opening reaction was also proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...