Phenanthrenequinone enantiomers with cytotoxic activities from the tubers of Pleione bulbocodioides†
Abstract
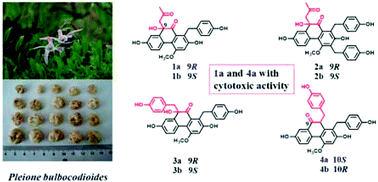
Eight new phenanthrenequinones (four pairs of enantiomers), named bulbocodioidins A–D (1–4), have been isolated from the ethanolic extract of tubers of Pleione bulbocodioides. Their structures, including absolute configurations, were determined by extensive NMR analysis combined with experimental and calculated ECD (electronic circular dichroism) data analyses. Compounds 1–4 possessed a 9(10)H-phenanthren-10(9)-one structure, which is a rarely reported instance from natural sources. Their possible biosynthetic pathway was discussed in the text. The cytotoxic effects of the isolated phenanthrenequinones were evaluated in several human cancer cell lines, and compounds 1a and 4a exhibited marked cytotoxic activities.


 Please wait while we load your content...
Please wait while we load your content...