Cu-Catalyzed carbamoylation versus amination of quinoline N-oxide with formamides†
Abstract
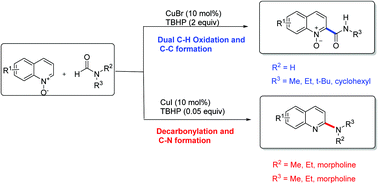
An efficient, direct carbamoylation and amination of quinoline N-oxides with formamides to access 2-carbamoyl and 2-amino quinolines has been developed through copper-catalyzed C–C and C–N bond formations via cross-dehydrogenative coupling reactions. The reaction proceeds smoothly over a broad range of substrates with excellent functional group tolerance under mild conditions. Mechanistic studies suggest that the reaction is initiated by formamide radical or decarbonylative aminyl radical formation in the presence of TBHP, according to the different substituent on the N atom of formamide.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...