Characterization of the flavin monooxygenase involved in biosynthesis of the antimalarial FR-900098†
Abstract
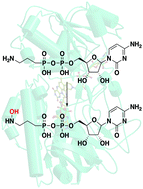
The latter steps in this biosynthetic pathway for the antimalarial phosphonic acid FR-900098 include the installation of a hydroxamate onto 3-aminopropylphosphonate, which is catalyzed by the consecutive actions of an acetyltransferase and an amine hydroxylase. Here, we present the 1.6 Å resolution co-crystal structure and accompanying biochemical characterization of FrbG, which catalyzes the hydroxylation of aminopropylphosphonate. We show that FrbG is a flavin-dependent N-hydroxylating monooxygenase (NMO), which shares a similar overall structure with flavin-containing monooxygenases (FMOs). Notably, we also show that the cytidine-5′-monophosphate moiety of the substrate is a critical determinant of specificity, distinguishing FrbG from other FMOs in that the nucleotide cofactor-binding domain also serves in conferring substrate recognition. In the FrbG-FAD+-NADPH co-crystal structure, the C4 of the NADPH nicotinamide is situated near the N5 of the FAD isoalloxazine, and is oriented with a distance and stereochemistry to facilitate hydride transfer.
- This article is part of the themed collections: Chemical Biology in OBC and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...