Synthesis of biurets via TMSNCO addition to 1-aminosugars: application in the de novo synthesis of dC oxidation products†
Abstract
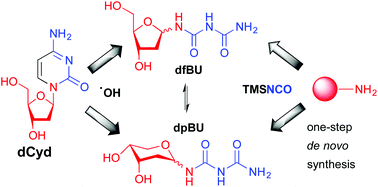
The reaction between 1-aminosugars and trimethylisocyanate (TMSNCO) was optimised as a one-step synthetic strategy for the synthesis of sugar biurets. This protocol was successfully applied to a number of 1-aminosugars, which exclusively provided the corresponding biurets in 67–99% yields. The new methodology was applied in the de novo synthesis of N1-(2-deoxy-α/β-D-erythro-pentofuranosyl)biuret (dfBU) and N1-(2-deoxy-α/β-D-erythro-pentopyranosyl)biuret (dpBU), two known DNA lesions arising from the hydroxyl radical induced decomposition of 2′-deoxycytidine (dCyd).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...