Synthesis of modified β-methoxyphenylalanines via diazonium chemistry and their incorporation in desoxycyclomarin analogues†
Abstract
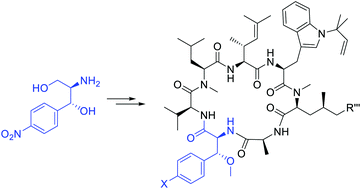
The marine natural products cyclomarins have remarkable anti-mycobacterial and antiplasmodial activities. The heptapeptic structure of this compound class comprisis four highly interesting non-canonical amino acids, including a rather unusual syn β-methoxyphenylalanine. To get a deeper insight into the structure–activity realtionship of cyclomarines, a straightforward protocol for the stereoselective synthesis of this building block was developed, based on diazonium chemistry.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...