Synthesis and biophysical characterization of oligonucleotides modified with O2′-alkylated RNA monomers featuring substituted pyrene moieties†
Abstract
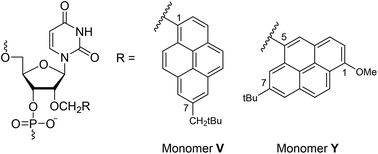
Over the past three decades, a wide range of pyrene-functionalized oligonucleotides have been developed and explored for potential applications in material science and nucleic acid diagnostics. Our efforts have focused on their possible use as components of Invader probes, i.e., DNA duplexes with +1 interstrand zipper arrangements of intercalator-functionalized nucleotides. We have previously demonstrated that Invader probes based on 2′-O-(pyren-1-yl)methyl-RNA monomers are energetically activated for sequence-unrestricted recognition of chromosomal DNA targets under non-denaturing conditions. As part of ongoing efforts towards delineating structure–property relationships and optimizing Invader probes, we report the synthesis and biophysical characterization of oligodeoxyribonucleotides (ONs) modified with 2′-O-(7-neo-pentylpyren-1-yl)methyl-uridine monomer V and 2′-O-(7-tert-butyl-1-methoxypyren-5-yl)methyl-uridine monomer Y. ONs modified with monomer V display increased DNA affinity (ΔTm up to +10.5 °C), while Y-modified ONs display lower DNA affinity and up to 22-fold increases in fluorescence emission upon RNA binding. Although these monomers display limited potential as building blocks for Invader probes, their photophysical properties render them of interest for diagnostic RNA-targeting applications.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...