Diastereoselective synthesis of 3,3-disubstituted 3-nitro-4-chromanone derivatives as potential antitumor agents†
Abstract
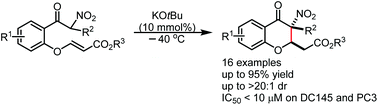
We report an efficient and highly diastereoselective protocol for the rapid construction of 3-nitro substituted 4-chromanones by an intramolecular Michael-type cyclization of α-nitro aryl ketones bearing unsaturated ester units. A catalytic amount of KOtBu was found to be crucial for the high diastereoselective control of this transformation. With this protocol, a series of 3,3-disubstituted 3-nitro-4-chromanones were synthesized in good to excellent yields with high diastereoselectivities and showed moderate to good in vitro antitumor activities, representing promising antitumor hits for further drug discovery.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...