Improved synthesis of 2,4,6-trialkylpyridines from 1,5-diketoalkanes: the total synthesis of Anibamine†
Abstract
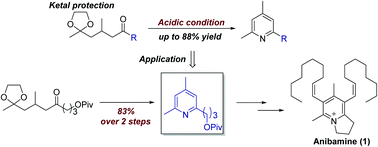
Many pyridine syntheses have been developed to date. In this study, we focused on pyridine synthesis with 1,5-diketone derivatives and hydroxylamine. Treatment of simple 1,5-diketoalkanes and hydroxylamine in basic or acidic conditions gave aldol adducts without any pyridine compounds. However, by screening the reaction conditions, we found that acidic conditions produced via the formation of oxime intermediates derived from 1,5-diketoalkanes allowed the formation of the corresponding pyridine derivatives. This is the first example of 2,4,6-trialkylpyridine synthesis from quite simple 1,5-diketoalkanes. In order to demonstrate the utility of the reaction, we demonstrated the synthesis of pyridine derivatives and the total synthesis of a 6-substituted pyridyl-natural product, anibamine. This was achieved by following the above methodology using a reported compound as the starting material to give the product in 12% yield.
- This article is part of the themed collections: Synthetic methodology in OBC and Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...