Facile amidinations of 2-aminophenylboronic acid promoted by boronate ester formation†
Abstract
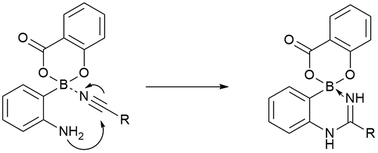
Amidine synthesis by amine addition to nitriles normally requires high temperatures or harsh catalysts. Here, we report that boronate esters can facilitate amidination of proximal amines with moderate heating. With amidines present in a number of drugs and the synthetic handle provided by the boron, this chemistry should find useful applications.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...