Synthesis of 1,2,5-oxathiazole-S-oxides by 1,3-dipolar cycloadditions of nitrile oxides to α-oxo sulfines†
Abstract
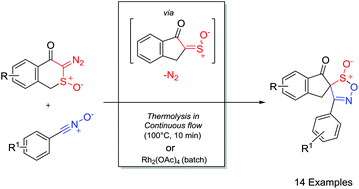
Synthetic methodology for the generation of novel 1,2,5-oxathiazole-S-oxides from cycloaddition of nitrile oxide dipoles with α-oxo sulfines generated in situ via the α-sulfinyl carbenes derived from α-diazosulfoxides is described. Experimental evidence and mechanistic rationale for the unanticipated interconversion of the diastereomeric 1,2,5-oxathiazole-S-oxide cycloadducts are discussed. Notably, using rhodium acetate as a catalyst at 0 °C under traditional batch conditions led to the selective formation and isolation of the kinetic isomers, while, in contrast, using continuous flow thermolysis, optimal conditions for the synthesis and isolation of the thermodynamic isomers were established.
- This article is part of the themed collections: Synthetic methodology in OBC and Carbenes in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...