Intramolecular trapping of ammonium ylides with N-benzoylbenzotriazoles in aqueous medium: direct access to the pseudoindoxyl scaffold†
Abstract
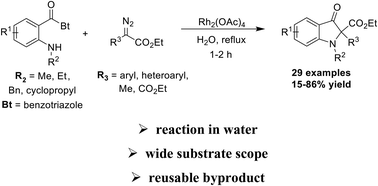
The present work documents an operationally simple, clean and practical method for accessing the 2,2-disubstituted indolin-3-one (pseudoindoxyl) scaffold. The rhodium carbenoid mediated reaction between N-o-alkylamino benzoylbenzotriazoles and aryl diazoacetates occurs smoothly in water and exploits the leaving group ability of the benzotriazole moiety to install the carbonyl function in the product. Other highlights of the methodology are a wide substrate scope and experimental practicality given the re-use of the benzotriazole byproduct for starting material preparation.
- This article is part of the themed collections: Synthetic methodology in OBC and Carbenes in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...