Stereoselective synthesis of chromane derivatives via a domino reaction catalyzed by modularly designed organocatalysts†
Abstract
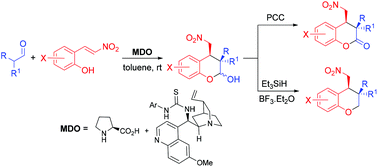
A highly enantio- and diastereoselective method for the synthesis of functionalized chroman-2-ones and chromanes was achieved by using an organocatalytic domino Michael/hemiacetalization reaction of aliphatic aldehydes and (E)-2-(2-nitrovinyl)phenols followed by a PCC oxidation and dehydroxylation, respectively. Using the modularly designed organocatalysts (MDOs) self-assembled from cinchona alkaloid derivatives and amino acids in the reaction media, the title products were obtained in good to high yields (up to 97%) and excellent diastereoselectivities (up to 99 : 1 dr) and enantioselectivities (up to 99% ee).
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...