Asymmetric α-alkylation of cyclic β-keto esters and β-keto amides by phase-transfer catalysis†
Abstract
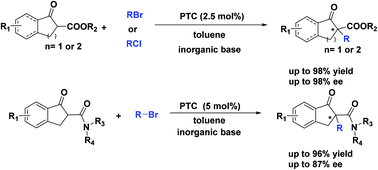
Without employing any transition metal, a highly enantioselective α-alkylation of cyclic β-keto esters and β-keto amides has been realized by phase-transfer catalysis. This improved procedure is applicable to different kinds of halides with cinchona derivatives and gives the corresponding products in excellent enantiopurities (up to 98% ee) and good yields (up to 98%). Moreover, the reaction was scalable and the phase-transfer catalyst was recyclable. This provided an alternative and competitive method to the asymmetric α-alkylation of β-dicarbonyl compounds.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...