Iron catalysed selective reduction of esters to alcohols†
Abstract
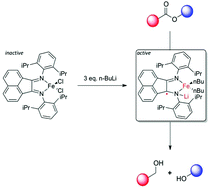
The reaction of (dppBIAN)FeCl2 with 3 equivalents of n-BuLi affords a catalytically active anionic Fe complex; the nature of the anionic complex was probed using EPR and IR experiments and is proposed to involve a dearomatized, radical, ligand scaffold. This complex is an active catalyst for the hydrosilylation of esters to afford alcohols; loadings as low as 1 mol% were employed.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...