Alkoxyallene-based syntheses of preussin and its analogs and their cytotoxicity†
Abstract
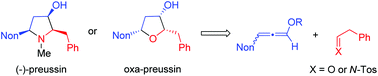
Short syntheses of oxa-preussin, racemic preussin and (−)-preussin are reported. Starting from a racemic 3-nonyl-substituted methoxyallene derivative, its lithiation and addition to phenylethanal provided the corresponding allenyl alcohol that was converted into two diastereomeric dihydrofuran derivatives by silver nitrate-catalyzed 5-endo-trig cyclization. The acid hydrolysis of the enol ether moiety gave heterocyclic ketones and subsequent highly stereoselective reductions with L-selectride furnished 2-benzyl-5-nonylfuran-3-ol derivatives in good overall yield. The major all-cis-diastereomer has the skeleton and relative configuration of preussin and is hence called oxa-preussin. An analogous sequence with the same allene, but an N-sulfonyl imine as the electrophile, finally led to racemic preussin. The stereoselectivities of the individual steps are discussed in detail. With an enantiopure 2-benzyl-5-nonylpyrrolidin-3-one intermediate the preparation of (−)-preussin with an enantiomeric ratio of >95 : 5 could be accomplished in a few steps. The sign of the optical rotation of this product finally proved the absolute configurations of its precursors and demonstrated that our chiral auxiliary-based route led to the antipode of the natural product. The cytotoxicity of several of the prepared heterocycles against MCF-7 tumor cells was investigated and five compounds, including racemic and enantiopure (−)-preussin, were identified as highly cytotoxic with IC50 values in the range of 3–6 μM.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...