A diversity of alkylation/acylation products of uracil and its derivatives: synthesis and a structural study†
Abstract
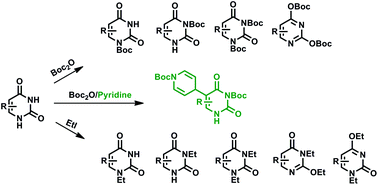
tert-Butyl dicarbonate (Boc2O) and ethyl iodide (EtI) reactions with uracil (U), thymine (T) and 6-methyluracil (6-MU) were performed following routine procedures in pyridine/DMF solvents and with DMAP as the catalyst. Among 20 synthesized compounds, a derivative of 6-methyluracil substituted by the Boc-pyridine moiety at the C5 position appeared unexpectedly. The NMR spectra confirmed the molecular structure of all uracil derivatives. Parallel quantum mechanical DFT calculations supported the experimental findings.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...