Crotonols A and B, two rare tigliane diterpenoid derivatives against K562 cells from Croton tiglium†
Abstract
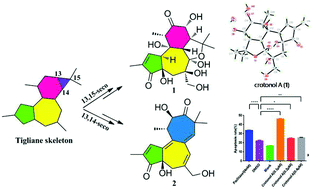
Crotonols A and B (1 and 2), two tigliane diterpenoids featuring a rare C-7/C-14 cyclized and novel 5/7/7-fused carbon skeleton, along with the known tigliane wallichiioid A, were isolated from the leaves of Croton tiglium. Their structures were determined through spectroscopic methods, X-ray crystallography and ECD analysis. To the best of our knowledge, crotonol B (2) represents the first example of 13,14-seco-tigliane diterpenoids. Crotonols A and B displayed strong cytotoxic activities against the K562 cell line with IC50 values of 0.20 and 0.21 μM, respectively. Furthermore, crotonol A promoted the apoptosis of K562 cells through the cleavage of PARP and the accumulation of bax as well as the degradation of bcl-2.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...