Multicomponent synthesis of pyroglutamic acid derivatives via Knoevenagel–Michael-hydrolysis-lactamization-decarboxylation (KMHL-D) sequence†
Abstract
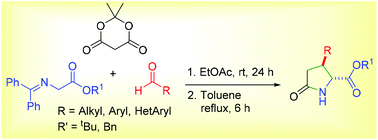
A novel and practical method for the synthesis of 3-substituted pyroglutamic acid derivatives is described. One pot multicomponent reaction of Meldrum's acid, aldehyde and Schiff's base followed an unprecedented chemoselective Knoevenagel–Michael-hydrolysis-lactamization domino sequence to afford 4-carboxy 3-substituted pyroglutamic acid derivatives under mild conditions. A carboxy intermediate formed appears to accelerate its own formation. The generality of the synthesis is exemplified by the use of a wide variety of aldehydes including enolizable aliphatic aldehydes, while substrates are stable under reaction conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...