Kinetic resolution of β-ketoesters with quaternary stereocenters via a carbene-catalyzed benzoin reaction†
Abstract
Chiral β-ketoesters bearing fully substituted carbon centers are important building blocks in organic synthesis. Mono-substituted ketoesters have been widely used to synthesize the above compounds through asymmetric additions or substitutions. The limitations of these protocols mainly exist in the substrate scopes, and α-methyl or α-fluoro-substituted β-ketoesters or acetyl acetates are frequently used owing to their relatively higher reactivity. To break through this limitation, we employed N-heterocyclic carbene-catalyzed kinetic resolution to achieve the access to enantioenriched β-ketoesters with quaternary stereocenters. Sterically more bulky groups such as benzyl, allyl, phenyl and cyclopropyl groups are all tolerated using this method.
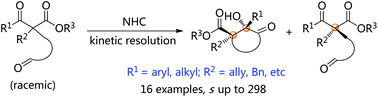
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...