Palladium-catalyzed intramolecular carboborylation of 1,3-diene and synthesis of ABCD ring of communesins†
Abstract
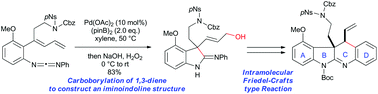
A palladium-catalyzed intramolecular carboborylation of 1,3-diene has been developed for the synthesis of iminoindolines with a quaternary carbon centre. This method was applied to a substrate bearing several functional groups to afford a complex iminoindoline, which was subsequently converted into an ABCD ring model compound of communesins via an intramolecular Friedel–Crafts-type reaction.
- This article is part of the themed collections: Total synthesis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...