Investigation of tetrazine reactivity towards C-nucleophiles: pyrazolone-based modification of biomolecules†
Abstract
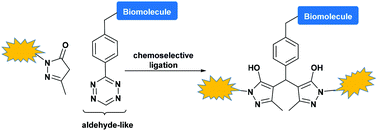
Chemoselective, biocompatible ligation reactions are the key components for efficient and modular access to biomolecular scaffolds. Tetrazine ligation leads to the formation of a mixture of isomers, which makes reaction monitoring, purification and characterization of conjugates difficult. We report herein a modified tetrazine ligation strategy based on the use of a pyrazolone coupling partner, which provides a single molecule conjugate.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...