Synthesis of 3-substituted 3-fluoro-2-oxindoles by deacylative alkylation†
Abstract
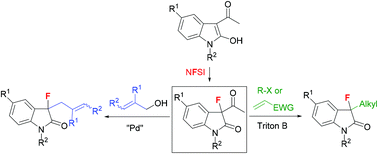
The fluorination of 3-acetyl-2-oxindoles with N-fluorobenzenesulfonimide under Lewis acid catalysis using Mg(ClO4)2 gives the starting compounds 3-acetyl-3-fluoro-2-oxindoles. These compounds are subjected to base-promoted deacylative alkylation (DaA) for the in situ generation of 3-fluoro-2-oxindole enolates under very mild reaction conditions using Triton B (1 equiv.) and alkyl halides and Michael acceptors as electrophilic reagents. The corresponding 3-alkylated-3-fluoro-2-oxindoles are obtained in good to very high yields. In addition, the palladium-catalyzed deacylative allylation is carried out with allylic alcohols using LiOtBu as the base and 6 mol% of Pd(OAc)2 and dppp, giving the resulting 3-allylated 3-fluoro-2-oxindoles in good yields. This methodology allows a simple synthesis of 3-alkylated-3-fluoro-2-oxindoles, which are difficult to obtain by other routes.


 Please wait while we load your content...
Please wait while we load your content...