An Fe stabilized metallic phase of NiS2 for the highly efficient oxygen evolution reaction†
Abstract
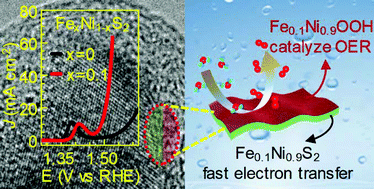
This work reports a fundamental study on the relationship of the electronic structure, catalytic activity and surface reconstruction process of Fe doped NiS2 (FexNi1−xS2) for the oxygen evolution reaction (OER). A combined photoemission and X-ray absorption spectroscopic study reveals that Fe doping introduces more occupied Fe 3d6 states at the top of the valence band and thereby induces a metallic phase. Meanwhile, Fe doping also significantly increases the OER activity and results in much better stability with the optimum found for Fe0.1Ni0.9S2. More importantly, we performed detailed characterization to track the evolution of the structure and composition of the catalysts after different cycles of OER testing. Our results further confirmed that the catalysts gradually transform into amorphous (oxy)hydroxides which are the actual active species for the OER. However, a fast phase transformation in NiS2 is accompanied by a decrease of OER activity, because of the formation of a thick insulating NiOOH layer limiting electron transfer. On the other hand, Fe doping retards the process of transformation, because of a shorter Fe–S bond length (2.259 Å) than Ni–S (2.400 Å), explaining the better electrochemical stability of Fe0.1Ni0.9S2. These results suggest that the formation of a thin surface layer of NiFe (oxy)hydroxide as an active OER catalyst and the remaining Fe0.1Ni0.9S2 as a conductive core for fast electron transfer is the base for the high OER activity of FexNi1−xS2. Our work provides important insight and design principle for metal chalcogenides as highly active OER catalysts.


 Please wait while we load your content...
Please wait while we load your content...