Investigating the origin of high efficiency in confined multienzyme catalysis†
Abstract
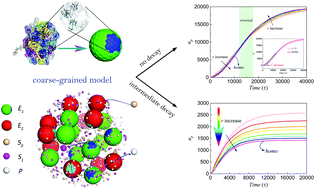
Biomimetic strategies have successfully been applied to confine multiple enzymes on scaffolds to obtain higher catalytic efficiency of enzyme cascades than freely distributed enzymes. However, the origin of high efficiency is poorly understood. We developed a coarse-grained, particle-based model to understand the origin of high efficiency. We found that a reaction intermediate is the key in affecting reaction kinetics. In the case of unstable intermediates, the confinement of multiple enzymes in clusters enhanced the catalytic efficiency and a shorter distance between enzymes resulted in a higher reaction rate and yield. This understanding was verified by co-encapsulating multiple enzymes in metal–organic framework (MOF) nanocrystals as artificially confined multienzyme complexes. The activity enhancement of multiple enzymes in MOFs depended on the distance between enzymes, when the decay of intermediates existed. The finding of this study is useful for designing in vitro synthetic biology systems based on artificial multienzyme complexes.


 Please wait while we load your content...
Please wait while we load your content...