Single molecule force spectroscopy reveals that the oxidation state of cobalt ions plays an important role in enhancing the mechanical stability of proteins
Abstract
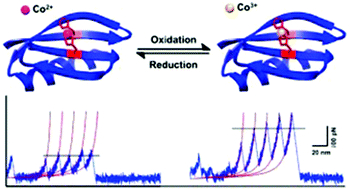
Engineered bi-histidine (biHis)-based metal chelation is a general and robust method to enhance the mechanical stability of proteins. Here we used single molecule force spectroscopy techniques to investigate the effect of binding of Co2+/Co3+ on the mechanical stability of an engineered biHis mutant of protein GB1, G6-53. We found that the binding of Co2+/Co3+ can lead to an enhancement of the mechanical stability of G6-53, but the degree of enhancement is drastically different. The binding of Co2+ can only lead to marginal enhancement of G6-53's mechanical stability, while Co3+ has a much stronger effect. This large difference is likely due to the large difference in thermodynamic stability and kinetic lability of Co2+ and Co3+ complexes. These results opened up new avenues towards fine tuning the mechanical properties of proteins.
- This article is part of the themed collection: 2019 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...