Titanium silicalite as a radical-redox mediator for high-energy-density lithium–sulfur batteries†
Abstract
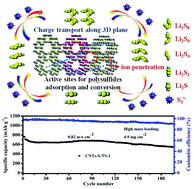
Lithium–sulfur (Li–S) batteries are receiving intense interest owing to their high energy densities, cost effectiveness, and the natural abundance of sulfur. However, practical applications are still limited by rapid capacity decay caused by multielectron redox reactions and complex phase transformations. Here, we include commercially available titanium silicalite-1 (TS-1) in carbon/sulfur cathodes, to introduce strong chemical interactions between the lithium polysulfides (LiPS) and TS-1 in a working Li–S battery. In situ UV-visible spectroscopy together with other experimental results confirm that incorporation of TS-1 mediators enables direct conversion between S82− and S3*− radicals during the discharge process, which effectively promotes the kinetic behaviors of soluble LiPS and regulates uniform nucleation and growth of solid sulfide precipitates. These features give our TS-1 engineered sulfur cathode an ultrahigh initial capacity of 1459 mA h g−1 at 0.1C. Moreover, the system has an impressively high areal capacity (3.84 mA h cm−2) and long cycling stability with a high sulfur loading of 4.9 mg cm−2. This novel and low-cost fabrication procedure is readily scalable and provides a promising avenue for potential industrial applications.


 Please wait while we load your content...
Please wait while we load your content...