Poly(triazolyl methacrylate) glycopolymers as potential targeted unimolecular nanocarriers†
Abstract
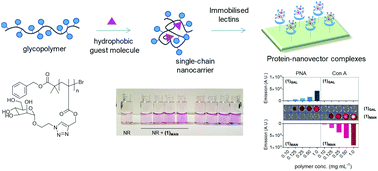
Synthetic glycopolymers are increasingly investigated as multivalent ligands for a range of biological and biomedical applications. This study indicates that glycopolymers with a fine-tuned balance between hydrophilic sugar pendant units and relatively hydrophobic polymer backbones can act as single-chain targeted nanocarriers for low molecular weight hydrophobic molecules. Non-covalent complexes formed from poly(triazolyl methacrylate) glycopolymers and low molecular weight hydrophobic guest molecules were characterised through a range of analytical techniques – DLS, SLS, TDA, fluorescence spectroscopy, surface tension analysis – and molecular dynamics (MD) modelling simulations provided further information on the macromolecular characteristics of these single chain complexes. Finally, we show that these nanocarriers can be utilised to deliver a hydrophobic guest molecule, Nile red, to both soluble and surface-immobilised concanavalin A (Con A) and peanut agglutinin (PNA) model lectins with high specificity, showing the potential of non-covalent complexation with specific glycopolymers in targeted guest-molecule delivery.


 Please wait while we load your content...
Please wait while we load your content...