Surface charged species and electrochemistry of ferroelectric thin films†
Abstract
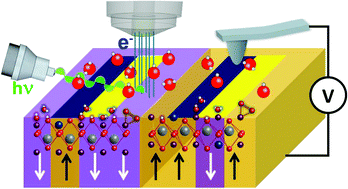
The combination of scanning probe microscopy and ambient pressure X-ray photoelectron spectroscopy opens up new perspectives for the study of combined surface chemical, electrochemical and electromechanical properties at the nanoscale, providing both nanoscale resolution of physical information and the chemical sensitivity required to identify surface species and bulk ionic composition. In this work, we determine the nature and evolution over time of surface chemical species obtained after water-mediated redox reactions on Pb(Zr0.2,Ti0.8)O3 thin films with opposite as-grown polarization states. Starting with intrinsically different surface chemical composition on the oppositely polarized films (as a result of their ferroelectric-dominated interaction with environmental water), we identify the reversible and irreversible electrochemical reactions under an external electric field, distinguishing switching and charging events. We find that while reversible ionic displacements upon polarization switching dominate screening in the bulk of the sample, polarization dependent irreversible redox reactions determine surface chemical composition, which reveals itself as a characteristic fingerprint of the ferroelectric polarization switching history.


 Please wait while we load your content...
Please wait while we load your content...