Crystal phase effect upon O2 activation on gold surfaces through intrinsic strain†
Abstract
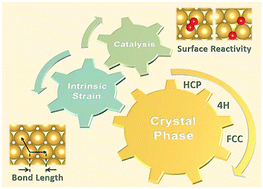
Crystal phase engineering is a promising strategy to tune the catalytic performance of metal nanomaterials. Generally, the crystal phase effect on catalysis is ascribed to distinct surface atomic arrangements of catalysts with different crystal phases. Here we show that even for similar surfaces, such as the close-packed surfaces, different crystal phases have considerably different surface reactivities due to their distinct intrinsic surface strains. Using first-principles calculations, we find that the close-packed surfaces of hexagonal close-packed (HCP) and double HCP (4H) gold have significantly smaller intrinsic strains (∼1.3%) than those of face-centered cubic (FCC) gold (∼2.3%). These distinct intrinsic surface strains result in various oxygen adsorption energies and O2 dissociation barriers on these close-packed gold surfaces, and the dissociation of O2 on different crystal phases and surfaces follows the Brønsted–Evans–Polanyi principle.


 Please wait while we load your content...
Please wait while we load your content...