Promoting Pt catalysis for CO oxidation via the Mott–Schottky effect†
Abstract
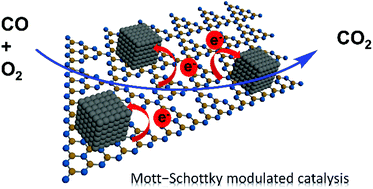
CO oxidation is an important reaction both experimentally and industrially, and its performance is usually dominated by the charge states of catalysts. For example, CO oxidation on the platinum (Pt) surface requires a properly charged state for the balance of adsorption and activation of CO and O2. Here, we present “Mott–Schottky modulated catalysis” on Pt nanoparticles (NPs) via an electron-donating carbon nitride (CN) support with a tunable Fermi level. We demonstrate that properly-charged Pt presents an excellent catalytic CO oxidation activity with an initial conversion temperature as low as 25 °C and total CO conversion below 85 °C. The tunable electronic structure of Pt NPs, which is regulated by the Fermi level of CN, is a key factor in dominating the catalytic performance. This “Mott–Schottky modulated catalysis” concept may be extended to maneuver the charge state on other metal catalysts for targeted catalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...