Chalcogenide vacancies drive the electrocatalytic performance of rhenium dichalcogenides†
Abstract
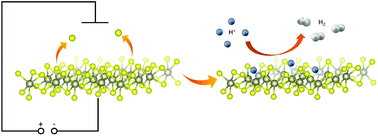
The hydrogen evolution reaction (HER) is one of the most promising ways to produce clean energy. However, its wide-spread use is hindered by the price of the state-of-the-art catalysts based on precious metals. Transition metal dichalcogenide (TMD) nanomaterials are a cheap alternative, but a relatively large portion of them remains unexplored in terms of the HER. Here we report the HER performance of rhenium disulfide and diselenide in an acidic environment. We used sodium naphthalenide as an exfoliation agent. In comparison with other TMDs, the degree of exfoliation was relatively low. On the other hand, rhenium disulfide and diselenide both exhibit high chemical stability towards oxidation even after the exfoliation. The reported HER performance of the exfoliated rhenium dichalcogenides was strongly dependent on electrochemical treatment and to further enhance the performance, we used a series of electrochemical pretreatments at potentials as high as −2 V in sulfuric acid. While the morphology of the samples remained unchanged, the surface was found out to be chalcogen deficient, pointing out the formation of chalcogen vacancies. Consequentially, the HER performance was substantially enhanced. These results were corroborated by theoretical calculations showing improved bonding of hydrogen by chalcogenide vacancies at the surface. Our results show that a very simple electrochemical procedure can be used to improve the electrocatalytic performance of rhenium dichalcogenides and, possibly, also other TMDs.


 Please wait while we load your content...
Please wait while we load your content...