Catalytic trends of nitrogen doped carbon nanotubes for oxygen reduction reaction
Abstract
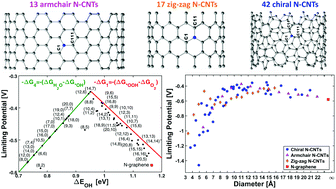
Replacing the state-of-the-art fuel cell catalyst platinum for a cheaper and abundant alternative would make the hydrogen economy viable. Both nitrogen-doped graphene and nitrogen-doped carbon nanotubes (N-CNT) have been shown to be capable of acting as a metal-free catalyst for the oxygen reduction reaction (ORR). Until now, most of the research has been focused on the nitrogen doping and less on the structure of the nanotubes. Here, density functional theory calculations are used to calculate trends in ORR catalytic activity of graphitic-N-doped CNTs of different sizes and chirality of selected tubes between (4,0) and (20,10). This includes 13 armchair tubes, 17 zig-zag tubes and 42 chiral tubes, or 72 N-CNTs in total. 22 tubes are predicted to have a lower overpotential than the platinum catalyst and 46 tubes have lower overpotential than nitrogen doped graphene. The most active tubes are (14,7), (12,6), and (8,8), and display an overpotential of around 0.35 V, or 0.1 V lower overpotential than predicted on Pt(111) with the same level of theory.


 Please wait while we load your content...
Please wait while we load your content...