Cu nanowire-catalyzed electrochemical reduction of CO or CO2†
Abstract
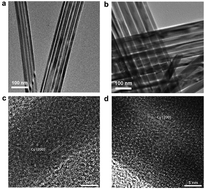
We prepared micrometer long Cu nanowires (NWs) of 25 and 50 nm diameters and studied their electrocatalysis for electrochemical reduction of CO/CO2 in 0.1 M KHCO3 at room temperature. The 50 nm NWs showed better selectivity than the 25 nm NWs, and catalyzed CO reduction to C2-hydrocarbons (C2H4 + C2H6) with a combined faradaic efficiency (FE) of 60% (C2H4 FE of 35% and mass activity of 4.25 A g−1 Cu) at −1.1 V (vs. reversible hydrogen electrode). The NW-catalyzed CO2 reduction is less efficient due to the extra CO2 to CO step required for the formation of C2-hydrocarbons. This experimental evidence combined with DFT calculations suggests that CO is an important intermediate and NWs provide a large Cu(100) surface for *CO hydrogenation (to *CHO) and *CO–*CHO coupling, leading to more selective reduction of CO than CO2 towards C2-hydrocarbons.
- This article is part of the themed collection: 2019 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...