Metallic 1T-phase MoS2 quantum dots/g-C3N4 heterojunctions for enhanced photocatalytic hydrogen evolution†
Abstract
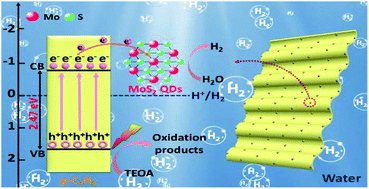
Recently, molybdenum disulfide (MoS2) has been regarded as an efficient non-precious-metal co-catalyst for photocatalytic hydrogen (H2) evolution, however, its inherent low-density active site and poor electron transfer efficiency have essentially limited its photocatalytic properties. Here we report that 1T-MoS2 quantum dots (QDs) can act as co-catalysts in assisting the photocatalytic H2 evolution to form heterostructures with g-C3N4 nanosheets (denoted as 1T-MoS2 QDs@g-C3N4). Benefiting from the abundance of exposed catalytic edge sites and the excellent intrinsic conductivity of 1T-MoS2 QDs, an optimized 1T-MoS2 QD@g-C3N4 composite (15 wt%) exhibits an extraordinary photocatalytic H2 evolution rate of 1857 μmol h−1 g−1 under simulated solar light irradiation, apparently 37.9 times higher than that of pure g-C3N4 NSs (49 μmol h−1 g−1). Meanwhile, the 1T-MoS2 QD@g-C3N4 composites exhibit a good stability in the cyclic runs for the photocatalytic H2 production. The high efficient photocatalytic activity and stability of the 1T-MoS2 QD@g-C3N4 composite is primarily attributed to the following reasons: (1) the introduction of 1T-MoS2 QDs results in a stronger light absorption capability in comparison with pure g-C3N4; (2) the tiny particle size of 1T-MoS2 QDs, in which edges and basal surface are catalytically active, provides a proliferated density of catalytically active sites; (3) 1T-MoS2 QD co-catalysts with metallic characteristics could act as efficient electron acceptors, which builds up a highly efficient pathway for photo-generated electrons from the CB of g-C3N4 NSs to 1T-MoS2 and thus realizes rapid spatial charge separation. The improved light harvesting ability, increased catalytically active sites, as well as increased separation of charge carriers could be responsible for the improved photocatalytic H2 evolution. This work will provide new insight for the design and fabrication of smarter, cheaper and more robust artificial photocatalysts for photocatalytic H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...