Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation†
Abstract
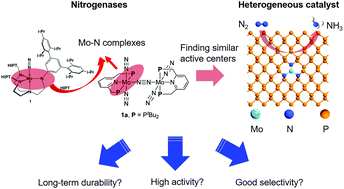
Ammonia (NH3) is one of the most significant industrial chemical products due to its wide applications in various fields. However, the production of NH3 from the electrochemical nitrogen (N2) reduction reaction (NRR) under ambient conditions is one of the most important issues that remain challenging for chemists. Herein, the candidacy of a series of molybdenum (Mo)-based single-atom catalysts (SACs) supported on N-doped black phosphorus (BP) as the electrocatalyst for the NRR has been evaluated by means of density functional theory (DFT) calculations. In particular, Mo1N3 has been found to chemically adsorb N2, and it exhibits the highest catalytic activity toward the NRR with an ultralow overpotential of 0.02 V via the associative distal mechanism, indicative of catalyzing the NRR under ambient conditions. Additionally, Mo1N3 shows the fast removal of the produced NH3 with a free energy uphill of only 0.56 eV and good stability of NRR intermediates. Moreover, the Mo-based SACs were demonstrated to be more selective to the NRR over the competing hydrogen evolution reaction (HER) process. These excellent features render Mo1N3 on BP as a compelling highly efficient and durable catalyst for electrochemical N2 fixation. Our results provide a rational paradigm for catalytic nitrogen fixation by SACs in two-dimensional (2D) materials under ambient conditions.
- This article is part of the themed collections: Editor’s Choice: Computational studies of nanomaterials for energy, catalysis and electronics and International Year of the Periodic Table : Single Atoms as Active Catalysts


 Please wait while we load your content...
Please wait while we load your content...