Facile synthesis of a bismuth nanostructure with enhanced selectivity for electrochemical conversion of CO2 to formate†
Abstract
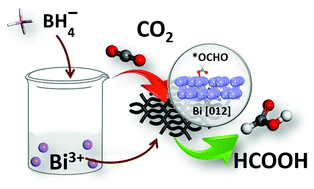
Electrochemically converting carbon dioxide (CO2) to formate offers a promising approach for energy conversion and storage. Bismuth is believed to be one of the promising candidates for CO2 electroreduction, but the poor selectivity and complexity of synthesis limit its real application on a large scale. In this work, a facile one-step-reduction method was developed to prepare a bismuth nanostructure in aqueous solution. Owing to its enhanced reactive sites and exposed crystal plane, the prepared Bi nanostructure exhibits excellent performance for CO2 electroreduction, which reaches the maximum faradaic efficiency for formate as high as 92% at a potential of −0.9 V versus a reversible hydrogen electrode. Additionally, the large current density and remarkable durability also reveal its high intrinsic CO2 electroreduction activity. The density functional theory calculation confirms that the formation of intermediate *OCHO that finally converts to formate is thermodynamically favorable on Bi high-index planes. We anticipate that such a facile synthesis strategy and excellent electrocatalytic performance of the Bi nanostructure will be easy to scale up, realizing its industrialization applications in CO2 electrochemical conversion.


 Please wait while we load your content...
Please wait while we load your content...