A biomimetic magnetosome: formation of iron oxide within carboxylic acid terminated polymersomes†
Abstract
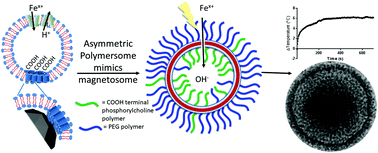
Bioinspired macromolecules can aid nucleation and crystallisation of minerals by mirroring processes observed in nature. Specifically, the iron oxide magnetite (Fe3O4) is produced in a dedicated liposome (called a magnetosome) within magnetic bacteria. This process is controlled by a suite of proteins embedded within the liposome membrane. In this study we look to synthetically mimic both the liposome and nucleation proteins embedded within it using preferential orientation polymer design. Amphiphilic block co-polymers self-assemble into vesicles (polymersomes) and have been used to successfully mimic liposomes. Carboxylic acid residue-rich motifs are common place in biomineralisation nucleating proteins and several magnetosome membrane specific (Mms) proteins (namely Mms6) have a specific carboxylic acid motifs that are found to bind both ferrous and ferric iron ions and nucleate the formation of magnetite. Here we use a combination of 2 diblock co-polymers: Both have the hydrophobic 2-hydroxypropyl methacrylate (PHPMA) block with either a poly(ethylene glycol) (PEG) block or a carboxylic acid terminated poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) block. These copolymers ((PEG113-PHPMA400) and (PMPC28-PHPMA400) respectively) self-assemble in situ to form polymersomes, with PEG113-PHPMA400 displaying favourably on the outer surface and PMPC28-PHPMA400 on the inner lumen, exposing numerous acidic iron binding carboxylates on the inner membrane. This is a polymersome mimic of a magnetosome (PMM28) containing interior nucleation sites. The resulting PMM28 were found to be 246 ± 137 nm in size. When the PMM28 were subjected to electroporation (5 pulses at 750 V) in an iron solution, iron ions were transported into the PMM28 polymersome core where magnetic iron-oxide was crystallised to fill the core; mimicking a magnetosome. Furthermore it has been shown that PMM28 magnetopolymersomes (PMM28Fe) exhibit a 6 °C temperature increase during in vitro magnetic hyperthermia yielding an intrinsic loss power (ILP) of 3.7 nHm2 kg−1. Such values are comparable to commercially available nanoparticles, but, offer the added potential for further tuning and functionalisation with respect to drug delivery.
- This article is part of the themed collection: International Year of the Periodic Table: Applications for magnetic materials


 Please wait while we load your content...
Please wait while we load your content...