Rapid formation of small mixed-valence luminescent silver clusters via cation-coupled electron-transfer in a redox-active porous ionic crystal based on dodecamolybdophosphate†
Abstract
Redox-active porous ionic crystals based on polyoxometalate (POM) were utilized to form and stabilize small mixed-valence luminescent silver clusters via cation-coupled electron-transfer (CCET) reactions. Reduction-induced ion-exchange between Cs+ and Ag+via CCET took less than 1 min to complete and consisted of two steps: electron transfer from reduced POM to Ag+ and the subsequent formation of a silver cluster, and diffusion of the silver cluster and exchange with Cs+. Notably, the simple ion-exchange took more than 24 h. The compound containing the silver cluster showed high affinity toward unsaturated hydrocarbon guests.
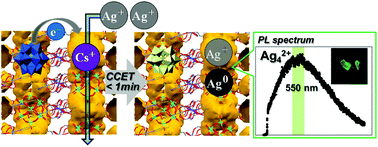


 Please wait while we load your content...
Please wait while we load your content...