MnO@graphene nanopeapods derived via a one-pot hydrothermal process for a high performance anode in Li-ion batteries†
Abstract
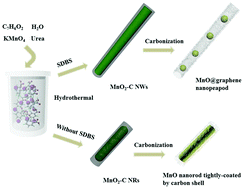
Although transition metal oxide–carbon (TMO–C) composites exhibit high Li storage capacity, the weak bonding between TMO particles and carbon mainly via van der Waals’ force and the limited internal void space result in poor rate capability and cycling performance. Herein, MnO@graphene nanopeapods are produced by calcination of hydrothermally-synthesized MnO2–C composites. The flexible graphene shells provide superior conductivity and excellent structural stability to the MnO cores, and the enough internal void space can significantly buffer the drastic volume expansion. The MnO@graphene nanopeapods exhibit high Li storage capacity (1168 mA h g−1 at 50 mA g−1 and 945 mA h g−1 at 500 mA g−1) at a voltage platform of ∼1.2 V, excellent rate capability (728 mA h g−1 at 1000 mA g−1 and 505 mA h g−1 at 3000 mA g−1), high initial coulombic efficiency (85.9%) and remarkable long-life cycling performance (undiminished after 1000 cycles). The MnO@graphene nanopeapods have been successfully used as the anode to assemble a full battery with LiFePO4 as the cathode. Our results provide a useful and rational strategy to design high performance graphene-supported MnO composites for Li ion batteries.


 Please wait while we load your content...
Please wait while we load your content...