Direct observation of the fast and robust folding of a slipknotted protein by optical tweezers†
Abstract
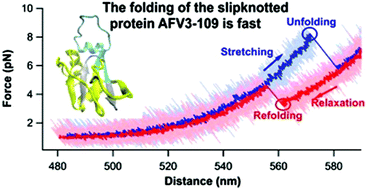
Understanding the folding mechanism of knotted and slipknotted proteins has attracted considerable interest. Due to their topological complexity, knotted and slipknotted proteins are predicted to fold slowly and involve large topological barriers. Molecular dynamics simulation studies suggest that a slipknotted conformation can serve as an important intermediate to help greatly reduce the topological difficulty during the folding of some knotted proteins. Here we use a single molecule optical tweezers technique to directly probe the folding of a small slipknotted protein AFV3-109. We found that stretching AFV3-109 can lead to the untying of the slipknot and complete unfolding of AFV3-109. Upon relaxation, AFV3-109 can readily refold back to its native slipknot conformation with high fidelity when the stretching force is lower than 6 pN. The refolding of AFV3-109 occurs in a sharp two-state like transition. Our results indicate that, different from knotted proteins, the folding of a slipknotted protein like AFV3-109 can be fast, and may not necessarily involve a high topological barrier.


 Please wait while we load your content...
Please wait while we load your content...