Tailoring the self-assembly of a tripeptide for the formation of antimicrobial surfaces†
Abstract
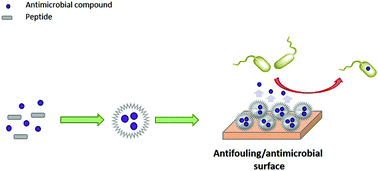
The accumulation of bacteria on surfaces is currently one of the greatest concerns for the management of proper healthcare systems, water and energy. Here, we describe the mechanism by which a single peptide forms two pH-dependent supramolecular particles that resist bacterial contamination. By using NMR and molecular dynamics (MD), we determined the structures of the peptide monomers and showed the forces directing the self-assembly of each structure under different conditions. These peptide assemblies change the characteristics of bare glass and confer it with the ability to prevent biofilm formation. Furthermore, they can adsorb and release active compounds as demonstrated with an anticancer drug, antibiotic and enzyme. This synergism and the detailed understanding of the processes are necessary for developing new sterile surfaces for healthcare systems, water purification devices, food packaging or any environment that suffers from biocontamination.


 Please wait while we load your content...
Please wait while we load your content...