A systematic study of the synthesis of cesium lead halide nanocrystals: does Cs4PbBr6 or CsPbBr3 form?
Abstract
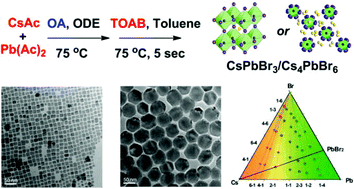
Cs4PbBr6 nanocrystals (NCs) have been recently studied as they can enhance the light emitting efficiency and stability of CsPbBr3 NCs. However, the synthesis of Cs4PbBr6 NCs is often accompanied by the generation of CsPbBr3 NCs, and less attention has been paid to how to exactly control their formation. Here, we investigated the key factors in deciding the final products in the hot-injection synthesis by a modified amine-free method. We found that the molarity ratio of Cs/Pb dominated the final products, while the amount of bromine had a relatively small effect. In addition, introducing a certain amount of oleylamine into the amine-free reaction leads to the formation of Cs4PbBr6, instead of CsPbBr3 NCs. This clearly shows that the protection ligand oleylamine also plays an important role in the formation of Cs4PbBr6 NCs. This well understood and fine control of the synthesis may inspire a new CsPbBr3@Cs4PbBr6 core–shell structure, with the same chemical elements but a different crystal phase in the core and the shell. This nanostructure would open a new avenue for enhancing the stability of perovskite nanocrystals.
- This article is part of the themed collection: Halide Perovskite Nanocrystals


 Please wait while we load your content...
Please wait while we load your content...