Facile synthesis, characterization and DFT studies of a nanostructured nickel–molybdenum–phosphorous planar electrode as an active electrocatalyst for the hydrogen evolution reaction†
Abstract
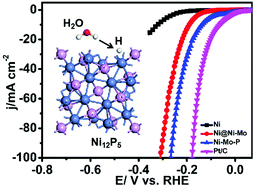
In this paper, we combined experimental and theoretical routes to develop a novel nanostructured nickel–molybdenum–phosphorous planar electrode as an efficient catalyst toward the hydrogen evolution reaction (HER). The HER activities of various Ni-based electrodes (Ni4Mo and Ni12P5) were evaluated not only experimentally but also by density functional theory. Meanwhile, the electrocatalytic performance of Ni–Mo–P prepared at different temperatures (500 °C, 600 °C, 700 °C, and 800 °C) was also explored. The results indicated that the sample prepared at 700 °C exhibited the best catalytic activity. The as-fabricated Ni–Mo–P electrode possessed lower overpotential, higher current density and a smaller Tafel slope than pristine modified Ni@Ni–Mo in 1.0 M KOH and also showed long-term stability. An overpotential as low as 276 mV could be achieved at 100 mA cm−2 H2 evolving current density, which was superior to those of most previously reported samples. After phosphorization treatment, the as-formed Ni12P5 played a crucial role in the activity enhancement. Density functional theory calculations revealed that Ni12P5 has a smaller |ΔGH*| value than Ni4Mo, further confirming that Ni12P5 shows better catalytic performance than Ni4Mo.


 Please wait while we load your content...
Please wait while we load your content...