Exploring the surface chemistry of cesium lead halide perovskite nanocrystals†
Abstract
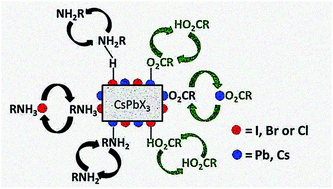
Colloidal nanocrystals (NCs) of cesium lead halide perovskites (CsPbX3, X = Cl, Br or I) are emerging as an exciting class of optoelectronic materials, but the retention of their colloidal and structural integrity during isolation, purification and handling still represents a critical issue. The impelling questions concerning their intrinsic chemical instability are connected to the dynamic nature of the bonding between the inorganic surface and the long-chain capping ligands. However, the key aspects of CsPbX3's surface chemistry that directly impact their stability remain elusive. In this contribution, we provide an in-depth investigation of the surface properties of differently composed CsPbX3 NCs, prepared by traditional hot-injection methods. The study, mainly relying on solution NMR spectroscopy, is backed up by elemental analysis as well as morphological, structural and optical investigations. We ascertained that the nature of the ligand adsorption/desorption processes at the NC surface is dependent on its elemental composition, thus explaining the origin of the instability afflicting CsPbI3 NCs. We also evaluated the effect of NC purification as well as of the degradation pathways involving the organic shell on the surface chemistry of CsPbX3 NCs. This study paves the way for new post-functionalization strategies for this promising class of nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...