Distinctively complete inhibition of fibrillation of serum albumins by methotrexate in vitro: experimental and modelling studies to understand the tuning of protein misfolding-related aggregations†
Abstract
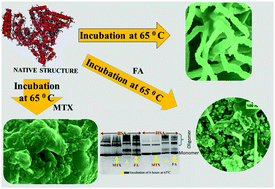
Observation of apparently never-ending lag-phase in the fibrillation of serum albumins (SAs) with low concentration of methotrexate (MTX) appears very promising and quite unique for clinical implications toward prevention of aggregation-related disorders. In the present work, we showcase that thermally induced bovine serum albumin (BSA) and human serum albumin (HSA) fibrillation is significantly inhibited by folic acid (FA), and completely by MTX. Surflex-docking, molecular dynamics (MD) simulation and aggregation-propensity studies provide a molecular level scenario that justifies the longer lag-phase in kinetics or high suppression of fibrillation by drugs.


 Please wait while we load your content...
Please wait while we load your content...