An umpolung reaction of α-iminonitriles and its application to the synthesis of aminomalononitriles†
Abstract
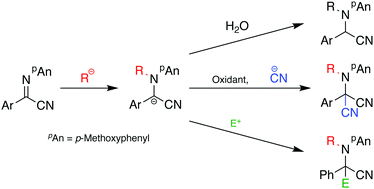
An umpolung N-alkylation reaction of α-iminonitriles with Grignard reagents affords the corresponding N-alkylated α-aminonitriles in good yields. Subsequent oxidation and cyanation of the N-alkylated products proceeds effectively to give aminomalononitriles in good yields, and the presence of dichlorodimethylsilane as an additive is crucial for obtaining the optimum yield. Furthermore, an electrophilic addition reaction of the intermediate halomagnesium vinylideneamide is shown to give the alkylated and acylated products in good yields.


 Please wait while we load your content...
Please wait while we load your content...